Reverse Osmosis (RO) is often a water purification technology which utilizes a semipermeable membrane to clear out ions, molecules, and larger particles from mineral water. In reverse osmosis, an applied pressure is utilized to overcome osmotic pressure, a colligative property, which is driven by chemical potential differences from the solvent, a thermodynamic parameter. Reverse osmosis can remove a number of dissolved and suspended species from water, including bacteria, and is utilized in both industrial processes as well as the production of potable water. The result is that the solute is retained about the pressurized side in the membrane as well as the pure solvent is capable to pass towards the other side. To be "selective", this membrane should never allow large molecules or ions over the pores (holes), but should allow smaller components in the solution (including solvent molecules) to give freely.
In the regular osmosis process, the solvent naturally moves from a location of low solute concentration (high water potential), by way of a membrane, to a place of high solute concentration (low water potential). The allure for the movement in the solvent may be the reduction inside the free energy from the system once the difference in solvent focus on either side of your membrane is reduced, generating osmotic pressure due to your solvent stepping into the more concentrated solution. Applying another pressure to turn back the natural flow of pure solvent, thus, is reverse osmosis. The process is much like other membrane technology applications. However, key differences are simply between reverse osmosis and filtration. The predominant removal mechanism in membrane filtration is straining, or size exclusion, therefore, the process can theoretically achieve perfect efficiency no matter parameters such as the remedy's pressure and concentration. Reverse osmosis also involves diffusion, making the procedure dependent on pressure, flow rate, along with conditions. Reverse osmosis is common for its use in h2o purification from seawater, taking off the salt as well as other effluent materials through the water molecules.
HISTORY
The technique of reverse osmosis through semipermeable membranes was observed in 1748 by Jean-Antoine Nollet. For the following 220 years, osmosis only agreed to be a phenomenon seen in the laboratory. In 1950, the University of California at Los Angeles first investigated desalination of seawater using semipermeable membranes. Researchers from both University of California at Los Angeles as well as the University of Florida successfully produced freshwater from seawater inside the mid-1950s, nevertheless the flux was too low for being commercially viable till the discovery at University of California at Los Angeles by Sidney Loeb and Srinivasa Sourirajan for the National Research Council of Canada, Ottawa, of approaches for making asymmetric membranes seen as an effectively thin "skin" layer supported atop a properly porous and a lot thicker substrate region with the membrane. John Cadotte, of FilmTec Corporation, found membranes with particularly high flux and low salt passage may very well be made by interfacial polymerization of m-phenylenediamine and trimesoyl chloride. Cadotte's patent within this process was the main topic of litigation and contains since expired. Almost all commercial reverse osmosis membrane is now manufactured by this method. By the end of 2001, about 15,200 desalination plants were operational or from the planning stages, worldwide.
In 1977 Cape Coral, Florida took over as first municipality inside the United States make use of the RO process over a large scale by having an initial operating capacity of 3 million gallons on a daily basis. By 1985, due to your rapid development in population of Cape Coral, the location had the biggest low pressure reverse osmosis plant inside the world, capable of producing 15 million gallons / day (MGD)
PROCESS
Reverse osmosis is often a natural process. When two solutions with assorted concentrations of your solute are separated by way of a semipermeable membrane, the solvent can move from low to high solute concentrations for chemical potential equilibration.
Formally, reverse osmosis would be the process of forcing a solvent from your region of high solute concentration by way of a semipermeable membrane with a region of low solute concentration by using a pressure in excess on the osmotic pressure. The largest and a lot important using reverse osmosis will be the separation of pure water from seawater and brackish waters; seawater or brackish water is pressurized against one surface from the membrane, causing transport of salt-depleted water throughout the membrane and emergence of potable h2o from the low-pressure side.
The membranes useful for reverse osmosis possess a dense layer within the polymer matrix—either your skin of an asymmetric membrane or perhaps an interfacially polymerized layer inside a thin-film-composite membrane—the place that the separation occurs. In most cases, the membrane is made to allow only water to pass through through this dense layer, while preventing the passage of solutes (for instance salt ions). This process mandates that a high pressure be exerted within the high concentration side on the membrane, usually 2–17 bar (30–250 psi) for fresh and brackish water, and 40–82 bar (600–1200 psi) for seawater, containing around 27 bar (390 psi) natural osmotic pressure that needs to be overcome. This process is most beneficial known for the use in desalination (treatment of salt along with minerals from sea water to get water), but as the early 1970s, they have also been employed to purify river for medical, industrial, and domestic applications.
DRINKING WATER
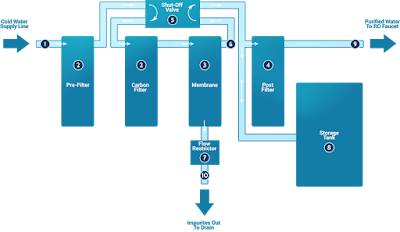
Around the entire world, household waters purification systems, including a reverse osmosis step, are commonly used in improving water for drinking and cooking.
Such systems typically add a number of steps:
a sediment filter capture particles, including rust and calcium carbonate
optionally, an additional sediment filter with smaller pores
an activated carbon filter to capture organic chemicals and chlorine, that could attack and degrade thin film composite membrane reverse osmosis membranes
a reverse osmosis filter, which can be a thin film composite membrane
optionally, a 2nd carbon filter to capture those chemicals not removed because of the reverse osmosis membrane
optionally an ultraviolet lamp for sterilizing any microbes which could escape filtering through the reverse osmosis membrane
latest developments within the sphere include nano materials and membranes
In some systems, the carbon prefilter is omitted, and cellulose triacetate membrane is employed. The cellulose triacetate membrane is susceptible to rotting unless protected by chlorinated water, as you move the thin film composite membrane is susceptible to breaking down intoxicated by chlorine. In cellulose triacetate membrane systems, a carbon postfilter is needed to get rid of chlorine in the final product, water.
Portable reverse osmosis water processors can be bought for personal water purification in numerous locations. To work effectively, the lake feeding about bat roosting units really should be under some pressure (40 pounds per sq . in . (280 kPa) or greater would be the norm).[7] Portable reverse osmosis water processors may be used by individuals who live in rural areas without clean water, far away through the city's water pipes. Rural people filter river or ocean water themselves, because device is easy to utilize (saline water may require special membranes). Some travelers on long boating, fishing, or island camping trips, maybe in countries in which the local water supply is polluted or substandard, use reverse osmosis water processors along with one or more ultraviolet sterilizers.
In the manufacture of bottled normal water, the stream passes by using a reverse osmosis water processor to eliminate pollutants and microorganisms. In European countries, though, such processing of natural drinking water (as defined with a European Directive[8]) is just not allowed under European law. In practice, a fraction from the living bacteria can and do move through reverse osmosis membranes through minor imperfections, or bypass the membrane entirely through tiny leaks in surrounding seals. Thus, complete reverse osmosis systems might include additional water treatment stages which use ultraviolet light or ozone to stop microbiological contamination.
Membrane pore sizes may vary from 0.1 to five,000 nm (4×10−9 to 2×10−4 in) determined by filter type. Particle filtration removes particles of merely one µm (3.9×10−5 in) or larger. Microfiltration removes particles of 50 nm or larger. Ultrafiltration removes particles of roughly 3 nm or larger. Nanofiltration removes particles of merely one nm or larger. Reverse osmosis is from the final class of membrane filtration, hyperfiltration, and removes particles greater than 0.1 nm.